Abdominocentesis
Daniel D. Smeak, DVM, DACVS, Colorado State University
Percutaneous sampling of peritoneal fluid with a needle or small catheter, called abdominocentesis (sometimes also known as peritoneocentesis or abdominal paracentesis), is a simple, rapid, and safe diagnostic method. When physical examination or imaging indicates moderate to large amounts of peritoneal effusion in a patient, sampling the fluid for cytologic, microbiological, or biochemical analysis often helps the clinician to quickly make a diagnosis and then to initiate timely and appropriate therapy. Abdominocentesis is most often performed to determine whether a patient needs exploratory celiotomy, particularly for early diagnosis of peritonitis or serious injury.
Before considering abdominocentesis, determine the relative value versus the risk of a blind puncture, particularly when a large vascular abdominal mass, an enlarged vascular organ, or a disorder affecting a distended hollow organ (such as a pyometra) is suspected, or in patients with severe coagulopathies. In these instances, consider ultrasound-guided sample collection if available. Abdominocentesis is best performed after plain radiography unless a rapid diagnosis needs to be made in a deteriorating patient. Free peritoneal air on radiographs may help the clinician identify hollow organ rupture or peritoneal perforation.
Procedure Pearl
Without prior imaging, any air introduced into the peritoneum during needle sampling can confuse the clinician, as it is impossible to determine if the free air is iatrogenic or caused by a ruptured hollow viscus or abdominal wall perforation.
In a large retrospective study of 129 dogs and cats with intraabdominal injury or disease, abdominocentesis had an overall diagnostic accuracy of 47% in dogs and cats (largely due to false-negative results), compared with 83% for catheter paracentesis, and 95% with diagnostic peritoneal lavage. The clinician must understand that false-negative results are reduced when larger amounts of free fluid are detected within the peritoneal space. False-negative results have also been reported with diseases confined to the retroperitoneal space and conditions resulting in only localized or walled-off fluid pockets. Diagnostic yield can be improved by ultrasound guidance to capture fluid within these pockets.
Procedure Pearl
Abdominocentesis has a high false-negative rate, especially when only a small amount of free peritoneal fluid is available to sample.
Step-by-Step: How to Perform Abdominocentesis
What You Will Need
In General
Clippers and blades
Antiseptic scrub
Sterile surgical gloves
Sampling Equipment
18- to 22-gauge 1.5-inch needles or 16- to 18-gauge over-the-needle catheters
Luer tip syringes (3-12 ml depending on the patient's size and amount of fluid needed for testing)
Sample containers
Serum "red-top" tubes (for biochemistry analysis)
EDTA "purple-top" tubes (for cytology, total protein content, red blood cell and total nucleated cell count)
Culturettes for bacterial culture
Glass slides for cytologic evaluation
Positioning & Skin Preparation
Abdominocentesis is usually performed in conscious animals with physical restraint. If the patient is intractable, judicious use of chemical sedation should be considered.
1. Clip and prepare the ventral abdominal area for aseptic fluid collection.
2. Infiltrate the proposed sites with a local anesthetic agent if a larger over-the-needle catheter is chosen for sampling.
3. For simple abdominocentesis, stand the patient or position it in sternal recumbency. Access the most dependent site on the abdomen. Alternatively, nonambulatory or unruly patients can be positioned in left lateral recumbency. First puncture the right side to help avoid hemorrhage or contamination of the sample from accidental aspiration of the spleen.
Author Insight
I experience fewer “dry taps” when I use a 16-gauge over-the-needle catheter into which several staggered, side port holes have been added with a scalpel blade. Multiple side ports reduce obstruction from loose apposing tissue, particularly when small amounts of intraabdominal fluid are expected.

Creating side ports in an over-the-needle catheter. Four or five staggered holes are made with a sharp no. 10 scalpel blade. Be sure the holes are no larger than one-third of the catheter circumference because larger holes may weaken the shaft and accidental breakage may occur when removing the catheter from the abdomen.
Productive Puncture Sites
Simple Abdominocentesis
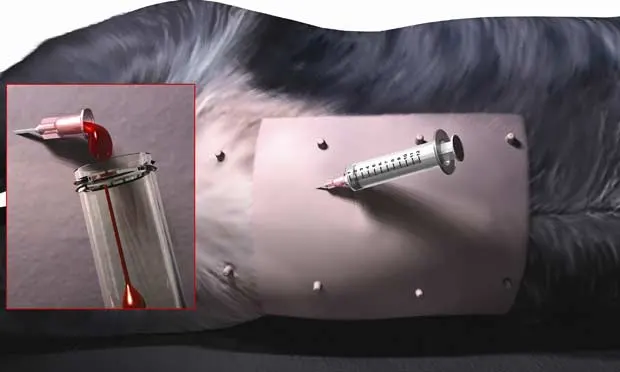
With the prepared animal in left lateral recumbency, insert the needle or fenestrated over-the-needle catheter just caudal to the umbilicus at or within 1 to 2 cm right of midline. Direct the needle toward the dependent side, slightly caudal toward the pelvis. The abdominocentesis shown here is positive. Fluid is flowing from the fenestrated catheter hub into a blood collection tube (Inset). If this approach is unsuccessful, attempt a second stick 2 to 4 cm cranial to the umbilicus on or slightly right of midline, with the needle angled in a slight craniodependent direction.

Author Insight
If simple abdominocentesis is unsuccessful and immediate results are important, a four-quadrant tap may be productive. Four sites for needle placement are used with the umbilicus as the center point.
Four-Quadrant Abdominocentesis
If simple abdominocentesis is unsuccessful and immediate results are important, a four-quadrant tap may be productive. With the prepared animal in dorsal or left lateral recumbency, insert the needle or catheters described above. Four sites for needle placement are used with the umbilicus as the center point: right cranial quadrant, left cranial quadrant, right caudal quadrant, left caudal quadrant.
Avoid the caudal superficial epigastric vessels during needle or catheter puncture. Stay away from an imaginary line drawn longitudinally along the nipples. Red Xs depict suggested quadrant tap sites. The caudal superficial and deep epigastric vessels lie paramedian in the neighborhood of a longitudinal line drawn between the nipples (green dashed lines). These vessels should be avoided during puncture.

Author Insight
Hemorrhagic effusion generally does not clot-blood from inadvertent splenic aspiration generally does.
Puncture Technique (Any Site)
With gloved hands, gently insert the needle (without a syringe attached) through the abdominal wall, and observe for any fluid within the hub.
Slowly rotating the needle, not "blind jabbing," may help fluid to escape through the needle.
When using a fenestrated over-the-needle catheter, observe for fluid escape once the stylet has been removed.
Collect any fluid that flows from the needle in the appropriate container.
If no fluid is obtained, attach an appropriately sized syringe and apply very gentle suction.
When one site is negative, repeat the needle puncture in another quadrant. A positive tap at any site completes the procedure.
Do not automatically accept that a negative abdominocentesis means little to no free abdominal fluid. Remember, between 5 and 6 mL/kg body weight of peritoneal fluid is required to achieve positive results with abdominocentesis in experimental dogs. If there is sufficient suspicion of abdominal effusion, ultrasound-guided needle sampling or diagnostic peritoneal lavage may be the next step. If diagnostic peritoneal lavage is planned, fluid can be infused and removed through the fenestrated over-the-needle catheter.
Author Insight
Applying undue suction with a syringe increases the incidence of obstruction of the needle with tissue and subsequent false-negative results.
Complications
Complications from abdominocentesis are limited and uncommon. Inadvertent puncture of the caudal superficial epigastric vessels may cause significant ventral abdominal bruising. Blind puncture can lead to hemorrhage or hollow organ laceration, but this complication is rare provided the patient is properly restrained. The most common complication is contamination of the sample with hemorrhage, or sampling of a hollow viscus, such as the bladder or intestine, and this may contribute to misdiagnosis. False-positive results can be expected in about 5% to 10% of attempts.
Evaluation of Peritoneal Fluid
A representative fluid sample should be thoroughly analyzed. The test results must be combined with the clinician's assessment of the animal's clinical condition and, in many cases, compared with peripheral blood values to obtain an accurate diagnosis of intraabdominal disease. The following is a list of tests that can be requested from the sample depending on the clinician's index of suspicion for a particular disease process. Appropriate sample collection, handling, and preparation are essential to obtain an accurate diagnosis. Refer to Connally's article (see Suggested Reading) for more information about sample preparation and which tests to consider for a variety of intraabdominal disease conditions.
Peritoneal Fluid Evaluation
General Fluid Analysis
Total protein
Red and white cell count
Specific gravity
pH
Cytologic Evaluation
White blood cell type and morphologic characteristics
Foreign matter
Intracellular organisms
Biochemical Analysis
Glucose
Creatinine
Urea nitrogen
Potassium
Cholesterol
Triglycerides
Bilirubin
Albumin
Globulin
Amylase and lipase
Lactate
Potassium
PCO2, PO2
Culture & Susceptibility
Aerobic and anaerobic bacterial culture