Wound Sampling for Culture & Cytology
J. Scott Weese, DVM, DVSc, DACVIM, FCAHS, Ontario Veterinary College, Guelph, Ontario, Canada

A properly collected swab. Only visible blood-tinged fluid is present, demonstrating that pus and necrotic debris were avoided.
Obtaining diagnostic specimens can be important for management of wound infections. Because wounds communicate with the external environment and body sites that harbor a commensal microbiota, proper technique is required to optimize sensitivity and specificity. A well-collected diagnostic specimen can help confirm the presence of bacterial infection, identify the causative organism, and provide critical antimicrobial susceptibility data. Poor sampling technique can complicate patient management by providing nondiagnostic or even misleading results.
Determining whether to collect a specimen and which diagnostic tests are necessary should be the first step. Although culture is useful in most situations, sampling for culture is less important (or should be avoided) in cases for which systemic antimicrobial therapy is not likely needed, a proper representative sample cannot be collected, contamination is likely, or sampling may compromise unaffected sites.
Bacterial culture is useful when the disease process is unclear and/or when systemic antimicrobials are to be used. Culture is particularly important when there is a greater likelihood that resistant pathogens are involved, such as infections that have failed empiric treatment or infections in patients with a history of antimicrobial treatment and/or hospitalization.
Although culture is often the focus of wound sampling, cytology should be considered whenever a specimen is being collected for culture. A specimen should always be submitted for cytology, which can be useful to confirm the presence of bacterial infection, rule out other causes, corroborate culture results, or identify fastidious organisms. Beyond routine aerobic culture and cytology, additional testing may be indicated in some situations, particularly chronic infections, infections that have not responded to appropriate treatment, and infections that are clinically atypical. This could include addition of special stains (eg, acid-fast, periodic acid–Schiff) or fungal culture. Anaerobic culture may be indicated in infections potentially associated with a penetrating wound or those that have a clinical appearance suggestive of anaerobic infection (eg, emphysematous tissue).
Specimen Types
When possible, tissue samples should be collected for culture. Although more invasive than fine-needle aspirates or swabs, tissue samples can have a higher sensitivity and specificity, particularly with deeper infections in humans.1,2
Fine-needle aspiration can be performed on deeper sites and are preferred over superficial swabs of draining tracts. The small volume collected and small area of the site sampled can limit sensitivity. Ideally, ≥2 samples should be collected, as positive results from >1 sample provide more convincing information of the clinical relevance of an isolated bacterial species.
Swabs are typically easier to collect and can be useful in many clinical situations. When performed properly, swabs are more prone to isolation of contaminants but yield fairly similar results as compared with other methods.3 Although tissue biopsy should be performed when possible, the following discussion focuses on collection of swabs, as this is the most common approach. Important concepts for wound sampling are presented in Important Considerations when Collecting a Specimen from a Wound.
Flocked swabs should be used when available, as they recover bacteria from infected sites as well as cotton- or rayon-tipped swabs but are more effective at releasing recovered bacteria into the culture medium.4
A properly collected swab often appears relatively clean, with only some blood-tinged fluid present (Figure).
Of note, fungal culture can be considered in any case, but fungal infections are uncommon. Fungal culture is more important in infections that do not respond as expected to antimicrobials, in infections with unusual clinical presentation or progression, or when cytology suggests fungal involvement.
Important Considerations when Collecting a Specimen from a Wound5
Sampling of the uncleaned surface of a wound is not recommended.
A specimen (eg, a swab or biopsy of clean but infected tissue), rather than a sample of a specimen (eg, pus), should be collected.
Healthy tissue—not necrotic tissue or pus—from the affected area should be sampled.
When possible, specimens should be collected prior to starting antibiotics.
Poor-quality specimens (eg, necrotic tissue, superficial swabs of pus) should not be submitted to the laboratory, as misleading information can be more harmful than no information.
Specimens should be labeled properly with the body site from which the specimen was collected. Only indicating “wound” is not adequate.
Sample Handling
Loss of viability (ie, false negatives) and contamination (ie, false positives) are of concern. Swabs for culture should be placed in an appropriate transport medium. Most commercial culture swab sets are effective at preserving viability of aerobic bacteria for the time typically required for a sample to reach the diagnostic laboratory. Anaerobes are more prone to death during transit, and either anaerobic transport media or combined aerobic/anaerobic transport media should be used if anaerobic culture is to be performed.
Swabs should be stored at room temperature if they will be processed by the laboratory within 2 hours. However, this will be difficult in most clinical situations; when delays will be encountered, swabs should be stored at refrigeration temperature (39°F [4°C]) until shipping and kept cool during shipping. Slides for cytology should be prepared immediately after collection. Ideally, the same swab should not be used for both cytology and culture, as contamination could occur while the swab is being rolled on a slide and preparing the cytology slide will remove some of the bacterial biomass.
Interpretation of Culture Results
Despite the use of optimal techniques, sampling will never be 100% sensitive and specific, and laboratory error (both technical error and abnormal behavior of bacteria in vitro) can occur. Any culture result must be carefully interpreted, with consideration of the body site, common pathogens, sample type, and the organisms that were isolated. Mixed infections can occur but are probably uncommon. Isolation of multiple organisms should be approached with caution, as one (or all) could be a contaminant. Although many commensals are opportunistic pathogens, isolation of bacteria that are common members of the commensal microbiota and typically of limited virulence (eg, coagulase-negative staphylococci, enterococci) is typically not clinically relevant.
When determining whether an isolated bacterium is likely clinically important, antimicrobial resistance is irrelevant. Resistance and virulence are different, and a multidrug-resistant bacterium is not more likely to be clinically relevant than a susceptible counterpart. Therefore, the bacterial species, infection site, and degree of bacterial growth—not the susceptibility pattern—should be considered.
STEP-BY-STEP
COLLECTION OF WOUND CULTURE SWABS
WHAT YOU WILL NEED
Sterile saline
Large syringe
Gauze
Gloves and, for some cases, a barrier gown
Culture swabs, ideally flocked swabs
Culture transport medium
Glass slides
Small surgical pack (optional)
STEP 1
Assemble all required supplies. Determine the degree of physical or chemical restraint that is necessary for proper sample collection.
STEP 2
Irrigate the wound with sterile saline (A). Debride any existing necrotic tissue. Then, using gauze, remove excess saline from the site (B), leaving a clean wound bed devoid of pus, debris, or necrotic tissue for sampling (C). If possible, let the site dry for ≈1 minute.
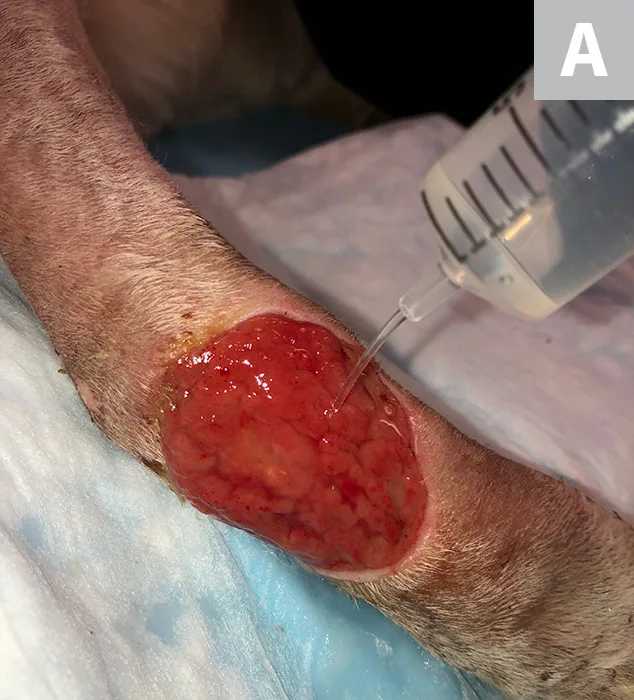
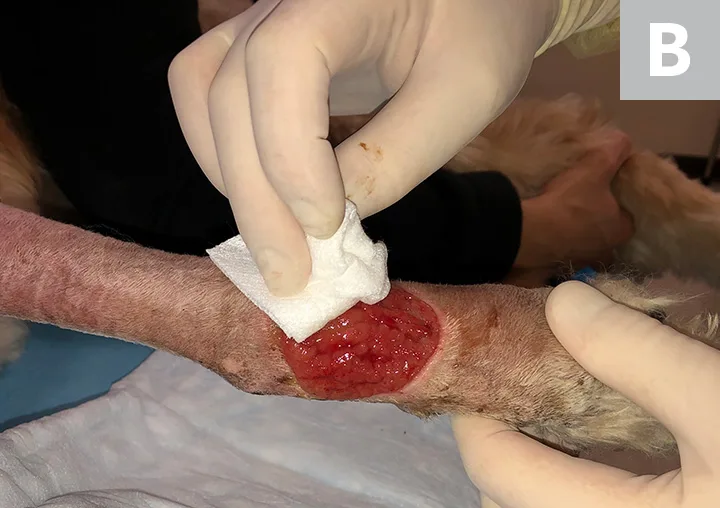
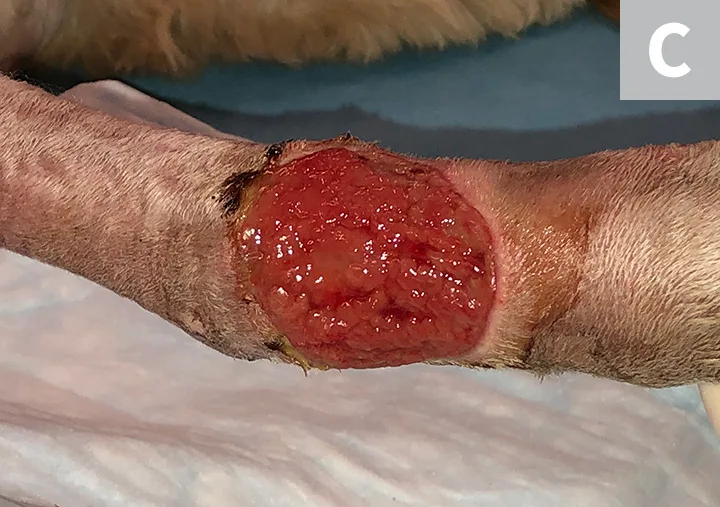
STEP 3
Replace gloves if they are contaminated and/or wet. Remove a sterile swab from its packaging, taking care not to contaminate it via contact with other surfaces. Rub the swab back and forth over ≈1 cm3 of viable tissue in the affected area for ≈5 seconds, taking care to avoid contact of the swab with sites that have a commensal microbiota (eg, skin and mucous membranes). Apply pressure to the swab to help express fluid from the wound bed.
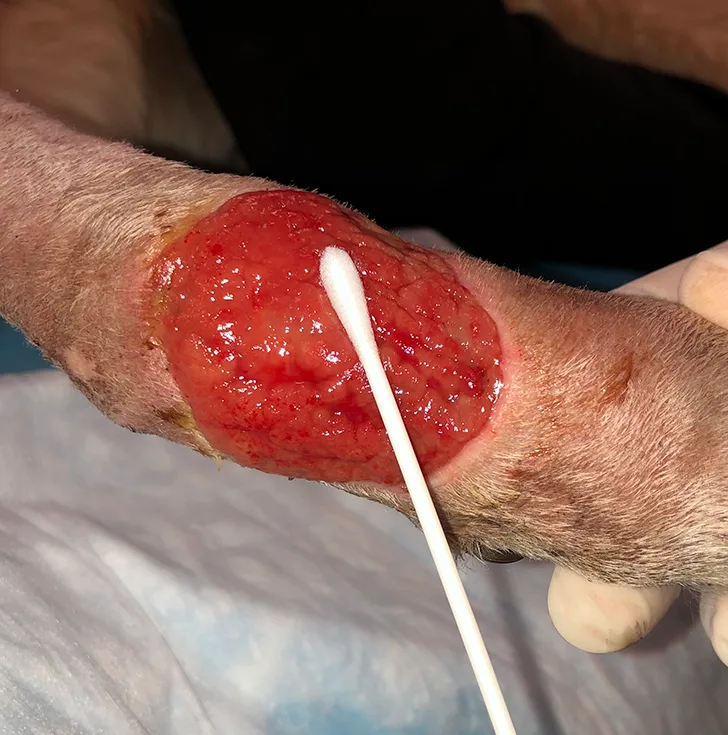
STEP 4
For culture specimens, place the swab into a transport medium and label the tube. Keep the swab at room temperature if it will be processed within 2 hours; otherwise, refrigerate the swab. Complete the laboratory submission form; include the specific sample location.
STEP 5
For cytology, roll a second swab onto a glass slide. Label the slide and allow it to air dry.

STEP 6
Indicate the swab site, disease process, species (along with any other relevant information), and patient history of antimicrobial therapy on the laboratory submission form.