Nasal Swabs to Detect Canine Influenza Virus
Jarod M. Hanson, DVM, PhD, DACVPM, DABT, University of Maryland
Ralph A. Tripp, PhD, University of Georgia
Stephen B. Harvey, DVM, MS, DACLAM, University of Georgia, United States Army Reserve
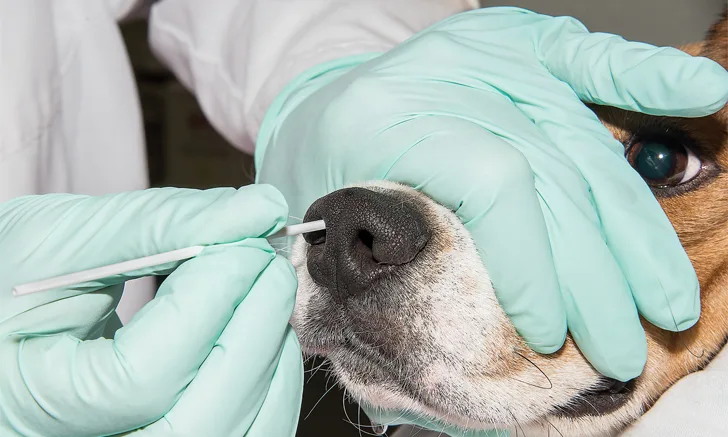
Nasal swabbing is the preferred antemortem sample collection technique for diagnosing acute influenza infection caused by canine influenza virus.
Although oropharyngeal swabs have been recommended in several species,1-3 nasopharyngeal swabs are as or more likely to identify influenza-infected animals and are the current standard for sample collection in multiple species (eg, humans, pigs).4-9 However, nasal swabs are preferred over nasopharyngeal or oropharyngeal swabs in dogs because of the inherent difficulty of obtaining high-quality swabs from the pharyngeal region caused by poor visibility of the target area, especially in large or brachycephalic breeds. In addition, the enhanced sensitivity of newer reverse transcription polymerase chain reaction (RT-PCR)-based influenza assays enable detection of small quantities of virus. Nasal swabs are also safer, as the dog can be muzzled during the procedure.
Sounding Board
A Letter to the Editor: Correct Nasal Swabbing Technique
Swabs can be used to detect influenza via polymerase chain reaction (PCR), virus isolation, and/or antigen-based enzyme-linked immunosorbent assay (ELISA) testing several days before development of a systemic immune response identifiable via traditional serologic methods; however, false-negative samples are common, even in dogs that later demonstrate influenza antibodies. False-negative results are a major problem with nasal swabs because of these factors:
Suboptimal technique
Virus inactivation and/or degradation due to improper swab and/or transport media selection
Lack of patient compliance during the procedure
Poor sample handling
Timing of sample collection relative to peak virus shedding
Additionally, as with the recent H3N2 canine influenza virus (CIV) outbreak, false-negative results may occur when a new strain of influenza emerges. Properly collected nasal swabs can be used for virus isolation, regardless of influenza type, allowing for detection and subsequent characterization of new virus isolates through sequencing and development of PCR assays. This decreases the time needed for diagnostic tests for a given influenza strain and to select an updated vaccine based on the newly identified strain(s).
Influenza replication occurs almost exclusively in epithelial cells lining the respiratory tract. Therefore, although swabs of the external nares may be useful for detecting diseases such as leishmaniasis, in which the agent lives in the mucocutaneous region,1,10 influenza sampling necessitates a much deeper swab technique to collect virus. To optimize CIV detection, swabs of the distal nasal cavity should be obtained.
Nasal swabs are preferred over nasopharyngeal or oropharyngeal swabs in dogs.
Swabs
Various types of nasal swabs are available; selecting an inappropriate type or material can have deleterious effects on the procedure and/or test outcome.4,5,7,8,11 Because the majority of patient samples will first be tested by PCR and then undergo testing with a virus isolation assay, only swabs acceptable for both diagnostic tests should be chosen. Sterile swabs should be used and may include:
Virus transport medium swabs
Polyester: standard or flocked-style swabs4
Culture swab systems for bacteria (only those with liquid media, not agar/gel)12,13
Other synthetic swabs; polyurethane foam swabs4
The US Centers for Disease Control and Prevention (CDC) does not recommend cotton or calcium alginate swabs for influenza diagnosis because of potential virus degradation and/or PCR interference.14
Avoid using wooden, paper, or aluminum wire handles because of the potential for residual chemicals to inactivate and/or degrade viral particles collected on the swab.15 Wooden handles are also more prone to break. Wire handles tend to bend during insertion, especially if the animal is not adequately restrained and the swab head is too small to adequately contact nasal epithelium; this can lead to poor virus recovery. Wire swabs may be useful, however, in pediatric or small-breed patients, in which small nasal passages may hinder introduction of a larger-diameter swab without significant trauma. Do not use wooden-handled swabs on live animals.
Restraint
Restraint is critical to prevent a swab from breaking in the nasal passage. It may take several veterinary team members to adequately restrain an unsedated dog to ensure its head remains still during swabbing. A swab that breaks off in the nasal passage can often be removed with a hemostat. Sedation should be used with caution in canine patients exhibiting respiratory compromise and/or distress.
Step-by-Step: Nasal Swab Technique
What You Will Need
Sterile 6-inch polyester (or similar) swabs with plastic handles
Sterile 5-mL red top, snap cap, or similar tube
Sterile isotonic saline or phosphate-buffered saline solution
Open-ended muzzle (not shown)
Sedative drugs (may be contraindicated)


STEP 1
With the dog muzzled,* place the swab parallel to the dog’s nose so that it extends to the medial canthus of the eye (Figure 1). This is the maximum depth the swab can be inserted into the nasal cavity. This distance varies with the length of the dog’s nose.
Author Insight
Avoid premoistening the swab, as this limits absorptive capacity and can result in lower sample recovery.
*Muzzle was removed for photographs to provide better visualization.
More About Influenza
Clinical influenza can be caused by many virus subtypes, all with unique species predilections. Crossover and subsequent adaptation of viruses among species remains an ongoing concern, although current canine influenza variants do not appear to readily cross back to other species.
Influenza variants and hosts include:
Influenza A virus: Humans, pigs, horses, dogs, cats, birds, ferrets, guinea pigs, mice, others
Influenza B virus: Humans, ferrets, seals
Influenza C virus: Humans, dogs, pigs, cows (also suggested as influenza D in cattle)16
Influenza A is further subdivided based on hemagglutinin (HA) and neuraminidase (NA) receptor types. Currently, 18 HA and 11 NA variants have been identified that can be combined in innumerable ways to yield unique viruses. Historically, H3N8 viruses originated in horses and spread to dogs, but the most recent canine virus, an H3N2 variant, appears to be of avian origin.17 It originated in 2007 in South Korea, where it was observed to infect dogs and cats; it has infected both species in the United States.18 Experimentally, dogs have been readily infected with both H5N1 and H6N1 avian-origin viruses. This indicates the potential for further influenza subtype introductions into the canine population.19
Conclusion
Nasal swabbing is an excellent diagnostic tool for identifying acute influenza infections, but it requires the right combination of swabs and technique to yield high-quality samples suitable for PCR, virus isolation, and other diagnostic tests.
Animal use was conducted under approval by the Animal Care and Use Committee of the University of Georgia, an institution accredited by the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) International.
CIV = canine influenza virus, ELISA = enzyme-linked immunosorbent assay, HA = hemagglutinin, NA = neuraminidase, PCR = polymerase chain reaction, RT-PCR = reverse transcription polymerase chain reaction,