Heartworms Resistant to Therapy?
Dwight D. Bowman, MS, PhD, Cornell University
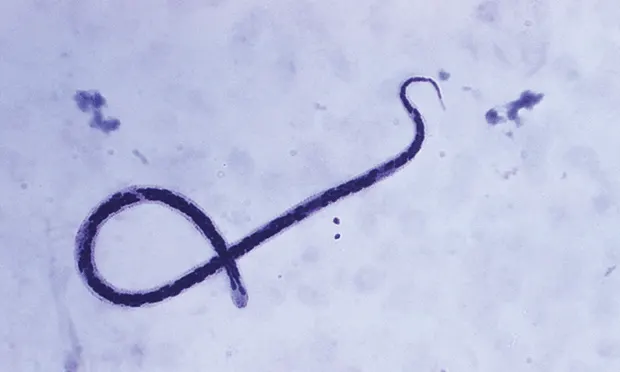
The goal of heartworm preventive therapy is to stop the infection of dogs with adult heartworms by targeting and killing third-stage and young fourth-stage larvae as well as microfilariae.
Preventive Heartworm Therapy in Dogs
As practitioners read this report, they should bear in mind 2 major factors that play a key role in how various drugs might lead to resistance or how they might differ in their effects on resistant worms:
Heartworm prevention
Major factors of resistance
First, heartworm preventives were designed and marketed at doses intended to prevent infection by killing third-stage and young fourth-stage larvae, not microfilariae. Thus, microfilariae may persist despite exposure to a macrocyclic lactone (ML); these drug-selected microfilariae can be transmitted between dogs by mosquitoes. This seems an unwise course of action if one wants to prevent selection of resistance through the effects of MLs on microfilariae.
Second, it should be remembered that some heartworm preventives had original targets other than heartworm and thus required higher minimum effective doses than were necessary to kill heartworms alone. Worms typically resistant to specific drugs will continue to demonstrate susceptibility to increased concentrations of the drugs until selection is so marked that the treatment becomes toxic to the host before the worms are killed. Dogs infected by third-stage larvae that develop to patency despite preventive therapy will harbor microfilariae.
NADA dose selection for dogs
Dose selection for heartworm prevention products has often been based on a minimum effective dose as determined by drug titration studies using experimentally infected dogs and sometimes based on doses for intestinal nematodes or fleas.
Heartgard 30 (NADA 138-412)
Minimum dose 6 µg/kg monthly
Interceptor (NADA 140-9150)
Final product dose 0.5 mg/kg based on efficacy for hookworms
Minimum effective dose 0.1 mg or above at 30 days postinfection appeared to have 100% efficacy
Revolution (NADA 141-153)
Minimum dose 6 mg/kg monthly
Dose originally based on efficacy against fleas on dogs for 30 days following single topical administration
ProHeart Tablets (NADA 141-015)
Minimum dose 3 µg/kg once monthly
ProHeart 6 Injectable (NADA 141-25)
Minimum dose 0.17 mg/kg
Excellent efficacy for hookworms at same dose
Drug resistance in other nematode models
In human medicine, control of the human filarial nematodes Onchocerca volvulus, Wuchereria bancrofti, and Brugia malayi has been driven differently. In this case, the goal has not been to prevent infection but to prevent transmission between people by suppressing microfilariae.1
Even though the campaign against human filariasis has been ongoing for more than 20 years with millions of doses administered without significant resistance, there are some recent indications that posttreatment microfilarial suppression may not last as long as originally thought; efforts do not appear to have hampered control in the field.2
What Is Resistance?
It’s tough to differentiate true resistance from other treatment failures
Resistance is defined as “a greater frequency of individuals within a population able to tolerate doses of a compound than in a normal population of the same species and is heritable.”3 Full reversion, as sometimes happens with removal of drug pressure, has not been observed in nematodes.4 Thus, detecting resistance in any nematode population is a concern. Often it is hard to pinpoint whether failure is a result of resistance or potential problems with recording, lack of compliance, underdosing, and reinfection.
The concern now is whether reported lack of efficacies (LOEs) with heartworm preventives are from resistance or a confluence of other factors that suggest apparent “resistance” but may be explained otherwise. This is the current ongoing debate.
The Mississippi Delta & Lack of Efficacy
Potential loss of efficacy: Noncompliance or resistance?
Anyone who has talked with practitioners from clinics in the area extending from Tennessee to Venice, Louisiana, has heard their claims that heartworm preventives are no longer protecting dogs from heartworm infection. On the other hand, it has been reported that a high percentage of failures have occurred secondary to lack of compliance5; this speaks to the continuing problem of trying to identify resistance in the field. Treatment failures may or may not accurately reflect an underlying issue of resistance.
Laboratory Studies & Case Presentations
Laboratory microfilariae isolates show reduced susceptibility
Blagburn and team
In vitro motility of microfilariae in the presence of macro-cyclic lactones (sponsored by Novartis Animal Health) In this Auburn University study, blood was collected from dogs that had purportedly been infected with heartworms while on preventive therapy; microfilariae were collected, purified, and examined for survival ability in different ML concentrations.6 Some isolates showed reduced susceptibility to MLs and were grown to the third larval stage in mosquitoes and used to infect dogs; microfilariae isolated from these infected dogs also demonstrated reduced ML susceptibility. This suggested that reduced susceptibility could be a form of genetically inherited resistance; however, this trait may or may not be linked to the ability of these microfilariae to grow to adulthood in dogs on preventive therapy. Now that Dr. Blagburn’s team has isolated these strains, experiments designed to test this relationship can take place.
ML = macrocyclic lactone, NADA = new animal drug application
Persistent microfilaremia in Hurricane Katrina rescue dog
Bourguinat and coworkers
Case report of persistent microfilaremia in a Hurricane Katrina rescue dog
At Cornell University, we have received reports of dogs with microfilariae that do not clear after treatment with Immiticide. Similarly, a case report recently described a Katrina rescue dog relocated to Canada and treated with Immiticide for heartworm infection on 2 separate occasions—5 months apart.7 The dog remained microfilaremic and antigen negative 8 months after the first treatment. The dog continued to be microfilaremic despite the second adulticide treatment and 2 doses of ivermectin (200 μg/kg). Eventually, the dog was treated every other week with milbemycin oxime, ultimately near the top of the preventive dose band at 1.1 mg/kg, and finally for 7 days at 2 mg/kg, followed a month later by 2 mg/kg for 8 days.
The dog remains antigen negative and microfilariae negative. These results seem to corroborate previous work evaluating refractory microfilariae.6 In this published case, it is unknown if the trait is heritable. Also, we have no proof that the phenotype of microfilarial resistance to MLs translates into resistance of third-stage larvae to MLs in dogs. This will require conclusive studies with Dr. Blagburn’s field isolates, but other work indicates that the 2 phenotypic traits—microfilarial resistance to MLs and resistance of larvae from mosquitoes to MLs (and hence continued susceptibility to preventive ML therapy in dogs)—may not be linked.
Polymorphism in heartworm genes: Molecular markers & treatment failures
McGill University and examination of molecular phenotypes
Drs. Bourguinat, Geary, and Prichard examined molecular phenotypes of many different nematodes and, more recently, heartworms to determine whether various molecular markers may be associated with treatment failures.12,13 Genes typically exhibit minimal polymorphism if changes in the gene are fatal to the worm; if there is no gene polymorphism, then determining the effects of drug selection on polymorphism frequency is of no value. In addition, selection can produce reduced gene polymorphism within a population.
Results revealed small differences in several of these molecular markers with random population distribution. The microfilariae from Blagburn’s group were markedly reduced in their polymorphism for the genes examined, suggesting that selection, possibly via ML, had driven worms toward genetic similarity in those genes. This would potentially hold true for third-stage larvae originating from these microfilariae. Reduced microfilarial polymorphism was also identified in the Canadian Katrina rescue dog whose microfilariae did not clear in the presence of high doses of milbemycin oxime.7 However, the genes from the new strain (ie, MP3) microfilariae are not unlike those of microfilarial isolates that are fully susceptible to macrocyclic lactones.14 Perhaps the resistance genotype seen in MP3 is unrelated to the genetic markers present in phenotypically ML-resistant microfilariae.
So, Do We Have Resistance?
The mechanisms of resistanceResistance can occur by several mechanisms, including mutation (ie, spontaneous or induced by such mutagens as irradiation or chemicals) or continued selection pressure (ie, repeated treatment of worms by a single drug, resulting in predominance of a particular phenotype in the population). The potential mechanism of resistance for heartworms is probably secondary to spontaneous mutation or via selection of a rare or uncommon phenotype.
Is MP3 a spontaneous mutation?
If microfilariae from dogs infected with third-stage larvae derived from MP3 microfilariae are capable of infecting dogs on ML preventives, this strain is resistant to MLs. It is unclear whether this potential resistance is new, induced, or selection based or represents inherent variability in the parasite population. Although multiple preventive doses may eventually be protective against MP3 infection, this strain does demonstrate reduced susceptibility to ML compared with other examined strains.
It does not matter whether the adult worms and microfilariae in Miss Piggy ever encountered MLs before. If the offspring can routinely infect dogs on preventives just like their parents, they are resistant worms. This could be the result of a single chance event occurring from the pairing of 1 male and 1 female worm (each with 1 copy of a recessive resistance gene) within Miss Piggy, resulting in microfilariae with a partially or relatively but not absolutely resistant phenotype. It may be that MP3 was a chance find by Dr. McCall’s laboratory.
Miss Piggy
The MP3 strain was isolated from a shelter dog named “Miss Piggy” from Georgia.
LOE = lack of efficacies, ML = macrocyclic lactone
Could MP3 have been selected by drug pressure?
It is also possible that resistance genes have persisted in a small D immitis population and that selection pressure has resulted in some worms with a resistant phenotype; some of these resistant worms my have found their way into Miss Piggy. MLs are derived from Actinobacteria spp; both filarial nematode and Actinobacteria spp ancestors were located in soil ecosystems, so genes supporting resistance to these products have likely provided a survival advantage to nematodes for eons. This suggests that resistance phenotypes were promoted by selection, already existed in the population, and never had an opportunity to reveal their survival potential until challenged. This might also be the case with a heritable trait within the MP3 worms from Miss Piggy; these worms did not have an opportunity to demonstrate survival in the presence of the drug until the isolate was captured by Dr. McCall’s group and studied in the laboratory.
How does drug selection work? Through selection pressure or adulticidal therapy using MLs?When parasitologists talk about anthelmintic resistance, they are usually considering the drug-induced selection of resistant forms. The selection of MP3 and possibly other heartworm strains in the United States with resistance to MLs could occur in at least 2 different ways: First, selection pressure may have been applied through regular preventive therapy; worms like MP3 (representing a small portion of the population) might sneak through in a few dogs on preventives. These infections might remain undetected if dogs with developed adults and microfilariae did not receive annual status checks, and the patent infections could then be spread by mosquito transmission. Second, adulticidal treatment of dogs with MLs (slow-kill or soft-kill) rather than Immiticide might select for populations of resistant circulating microfilariae that are spread to new dogs by mosquitoes. In the first case, selection is at the level of third- and fourth-stage larvae; in the second case, selection would occur at the level of microfilariae.
Closing Remarks
We do not know if the strains isolated by Dr. Blagburn (using microfilariae surviving in relatively elevated levels of MLs) can develop to adults in dogs on preventive therapy and do not know whether they can survive after a single treatment (as did MP3 in trials). Current data suggest that the microfilarial assay developed by Dr. Blagburn may measure lack of susceptibility in microfilariae from dogs; however, this may not correlate with increased third-stage larval survival in the presence of these anthelmintics. In addition, molecular characterization of Dr. Blagburn’s microfilarial isolates with reduced ML sensitivity was different from those of MP3.
If MP3 offspring are found to behave the same in similar drug efficacy trials as the original isolate, the trait is heritable and resistance could be assumed. There may be resistance-associated phenotypes naturally occurring at some low level in the D immitis population. As more work is done, we can only hope to have answers in the not-too-distant future.
The issue of resistance is being carefully examined in many research settings. The heartworm life cycle is long; it takes at least 6 months to develop adult worms with circulating microfilariae in a dog and then another 6 months to determine whether microfilariae develop into resistant adult worms after they have grown to the third-stage in mosquitoes and are used to infect additional dogs.
Worms, unlike bacteria, require sexual recombination through male–female mating to produce offspring. What if there is only 1 adult male in the MP3 population that has the resistant trait? There is a good chance that such a male may not develop in the group of 30 to 50 worms in the next infected dog. At this writing, we do not know whether the worms from MP3 infection can produce microfilariae and whether infections develop and persist in the face of preventive therapy.
For now, a prudent approach remains vigilance in testing dogs before starting them on heartworm prevention and vigilance in testing dogs annually for heartworms to avoid letting any MP3 (or other potentially resistant strain) slip by, live, and produce microfilariae while the dog is on prevention.
Administration of doxycycline to dogs might suppress infectivity to the next canine host of third-stage larvae developed from the original dog’s microfilariae.15 Recent data emphasize the need for additional research and for veterinarians to urge clients to keep pets current on monthly heartworm prevention, to practice careful recordkeeping, and to continue reporting all LOEs to the FDA.
Laboratory-Based Studies & Drug Development
First laboratory study on treatment efficacy
Work with Revolution
Many practitioners may forget that in July 2004 Pfizer Animal Health released additional experimental studies suggesting that some dogs treated with Revolution harbored 1 or 2 adult heartworms 5 months after laboratory challenge, while all untreated control dogs exhibited substantial worm burdens (14–43 adult worms per dog). This was the first report of laboratory studies in which heartworms developed in dogs given a single dose of a preventive 30 days after inoculation with infective larvae from a laboratory strain.
Efficacy of milbemycin oxime-containing ML
The development of Trifexis
In 2011, 2 papers appeared on the development of a new milbemycin oxime–containing product, Trifexis, by Elanco Animal Health.9,10 Dose confirmation testing protocols were conducted for an FDA CVM–approved label claim for heartworm prevention. Dogs were inoculated with 50 infective D immitis L3 from experimentally infected mosquitoes and either received a single dose of the preventive 30 days postinoculation or were not treated. Two separate and recently identified heartworm isolates were tested; one isolate was fully susceptible to the ML, with 100% prevention after administration of a single dose 30 days post-L3 inoculation. However, efficacy against the second isolate was lower than 100%. In this work, 3 of 10 infected dogs treated with Trifexis had 3 worms at necropsy, 1 dog had one worm, and 2 dogs had 2 worms; the strain MP3 isolated from a dog named Miss Piggy from Georgia was maintained in dogs by Dr. McCall at TRS Labs, Inc.
At this point, Elanco continued its research and an additional study evaluated the effectiveness of currently marketed ML products, including Heartgard Plus Chewables for Dogs (ivermectin) and Interceptor Flavor Tabs for Dogs and Cats (milbemycin). In this study, dogs were infected with 50 third-stage MP3 heartworm larvae from mosquitoes and treatment initiated 30 days later, with necropsy 153 days postinoculation.9 A single worm was found in 1 dog in each of the Heartgard Plus and Interceptor treated groups of dogs (14 dogs in each group), confirming that neither product was 100% efficacious in preventing infection.
For Trifexis, additional studies using the new MP3 strain (MP3 laboratory strain; TRS Laboratories, Athens, Georgia) looked at the effects of multiple treatments at 30 and 60 days postinfection and at 30, 60, and 90 days postinfection. In the 10 dogs treated twice with Trifexis, there was a single worm present at necropsy at 153 days postinfection (1 worm in 1 dog), and no worms in the dogs treated 3 times. Thus, the Trifexis label reads, “For heartworm prevention, give once monthly for at least 3 months after exposure to mosquitoes.”
Efficacy of MLs challenged, prompting additional research
MP3 and Advantage Multi versus Heartgard Plus, Interceptor Flavor Tabs, and Revolution
In 2011 as part of product development, Bayer Animal Health disclosed working on a new ML-containing preventive (2 formulations of ivermectin-containing products with target minimum doses of 6 μg/kg and 9 μg/kg; personal communication as to dose bands used). This work involved the MP3 strain.11 The results suggested that the MP3 laboratory strain had decreased susceptibility to typically efficacious ivermectin preventives, resulting in additional research evaluating the efficacy of 4 commercial monthly preventive products against MP3.
Dogs were each infected with 100 MP3 third-stage larvae and treated 30 days later with 1 of 4 preventive products. Necropsies were performed 150 days after initial infection. Worms were recovered from dogs in all groups except for Advantage Multi. In these single treatment studies, only Advantage Multi was 100% efficacious in preventing development of MP3 to adulthood, suggesting that protection of all dogs with all products was not uniform for the MP3 isolate.
MP3 Resistance Phenotype
It is unclear whether the resistance phenotype of MP3 is conserved in the worm genome in subsequent generations and whether it is inherited by offspring when the worms mate. This will not be elucidated until worms from a dog infected with third-stage MP3 larvae or the isolates maintained by Dr. Blagburn grow to adulthood and produce microfilariae that are used to infect mosquitoes, which ultimately infect a new set of dogs, and are then challenged by treatment 30 days postinfection.