Interventional Radiology: A Trend in Veterinary Medicine
Chick Weisse, VMD, DACVS, Animal Medical Center
Minimally invasive surgery has revolutionized human medicine, resulting in reduced morbidity and mortality, shorter hospitalization times, and the ability to manage otherwise untreatable conditions.
More than simply providing the ability to perform the same surgical procedures with smaller incisions and faster recovery times, interventional radiology (IR) techniques can provide new approaches to a variety of disorders in veterinary patients with otherwise few options.
Minimally invasive surgery is best known to veterinarians in the form of endoscopic, laparoscopic, and thoracoscopic procedures, in which surgery is performed with the aid of video assistance. A previous article (Interventional Endoscopy: The Essentials, October 2013) described some of the new procedures performed using interventional endoscopy.1 Less commonly known, but equally important, image-guided procedures using fluoroscopic guidance now offer similar opportunities to treat difficult conditions.
Contemporary imaging techniques, including ultrasonography and fluoroscopy, are used for the selective access of vascular and other structures for therapeutic delivery of various materials. The following introduces veterinary IR and some of the currently offered procedures.
History & Background
After the initial description of percutaneous arterial catheterization in 1953,2 angiography emerged as a diagnostic modality that was essential for medical diagnosis and had vast therapeutic application in humans.
IR techniques have been adapted for virtually every body system and can be used in patients of all sizes. As these techniques have expanded, there are now further subspecializations into areas such as interventional oncology (IO), which is called the fourth pillar of human oncology because of the vast array of new treatment options.
Advantages & Disadvantages
IR techniques can be more advantageous than open traditional techniques; they are minimally invasive and can result in diminished perioperative morbidity and mortality, decreased anesthesia times, shorter hospitalization, and potential reductions in cost.
Following the introduction of less expensive veterinary-focused devices, the majority of these procedures are no more expensive than their open surgical counterparts. Many clinical veterinary conditions may not be amenable to standard therapeutic intervention, and some IR techniques, including tumor chemoembolization or palliative stenting of malignant obstructions, may provide alternate therapeutic options.
IO involves the use of these techniques to treat cancer patients that would otherwise have no good options. As veterinary cancer patients are typically diagnosed at later stages of disease and treated with lower doses of chemotherapy than humans, IO is likely to play an increasingly important role in the management of veterinary cancer patients.
IR does have clinical disadvantages, including the need for specialized equipment, large initial equipment investment, and specific expertise. Fortunately, there are training laboratories nationwide that can teach veterinarians these techniques. In addition, many university teaching hospitals and large referral practices are offering these techniques to an increasingly wide population of animals.
Equipment & Technique
The majority of minimally invasive IR procedures—many of which are performed through small entry points in the skin or via natural orifices through catheters—can be performed in clean angiography suites or hybrid procedural rooms, although sterile operating rooms are still preferred. Sterile technique and surgical scrubbing with full surgical outerwear are required; lead gowns and thyroid shields are utilized, as radiation exposures during fluoroscopic procedures can be substantial.
A full implementation of radiation safety guidelines is necessary. A traditional fluoroscopy unit is sufficient for the majority of common veterinary IR procedures. The ability to move the image intensifier in the C-arm fluoroscopy unit allows for multiple tangential views of the patient while minimizing patient mobility.
Common Procedures
Tracheal Stenting
Tracheal collapse is characterized by clinical signs such as cough, raspy breathing, and dyspnea. Signs can often be treated palliatively with cough suppressants, bronchodilators, sedatives, tranquilizers, or NSAIDs, yet adequate control of disease is not always possible. Candidates for surgery include patients in which initial conservative medical management has failed. However, excessive morbidity and mortality rates have been reported for the extraluminal ring prosthesis open surgical procedure.3
Clinical improvement can be achieved in 75% to 90% of animals treated with self-expanding metallic stents (Figure 1), an IR procedure.4 Immediate adverse events were mostly minor; however, a perioperative mortality rate of about 10% was reported. Longer-term adverse events included shortening of the stent, fracturing of the stent, progressive tracheal collapse, and excessive granulation tissue.
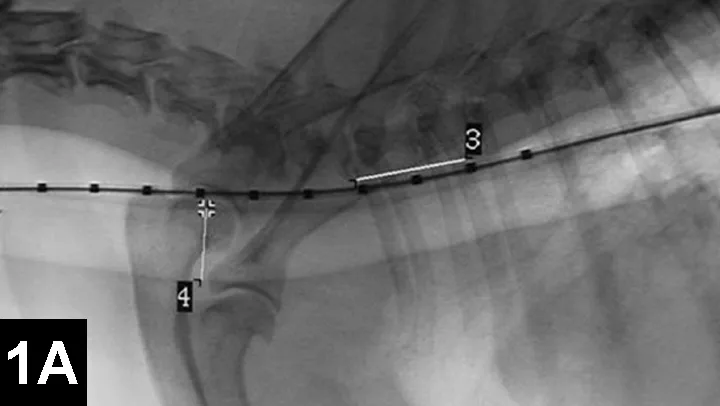
Figure 1A: Serial lateral fluoroscopic images of a dog with tracheal collapse.
A positive pressure ventilation demonstrates maximal dilation of the trachea. A marker catheter in the esophagus is used to account for radiographic magnification.
Palliative Stenting for Benign or Malignant Obstructions
Veterinary patients may present with severe intractable strictures of the esophagus, urethra, or other structures that do not respond to multiple dilation techniques. In addition, malignant obstructions of the urinary tract, GI tract, or cardiovascular system can result in clinical signs that are not amenable to traditional therapies.
Presenting signs may be affected by location of the tumor and secondary local effects instead of the systemic effects of the tumor itself. For example, malignant obstructions of the urinary tract are typically caused by transitional cell carcinomas or prostatic tumors. Affected patients are often presented with signs associated with obstruction of the urinary tract.
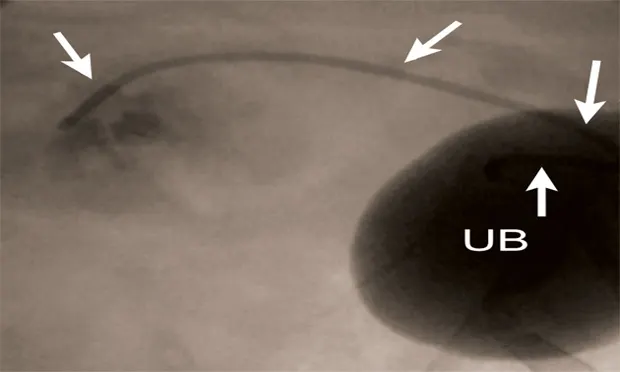
IR techniques that involve placement of an intraluminal stent to alleviate signs associated with malignant obstructions have been described in veterinary patients (Figure 2).5 The author has performed multiple palliative stenting procedures in the urinary tract and upper and lower GI tracts and has relieved luminal obstructions caused by neoplasia or intractable benign strictures in many different sizes of animals, including ferrets.5-11 The IR techniques were fast, safe, and effective. Complications were minor and uncommon.
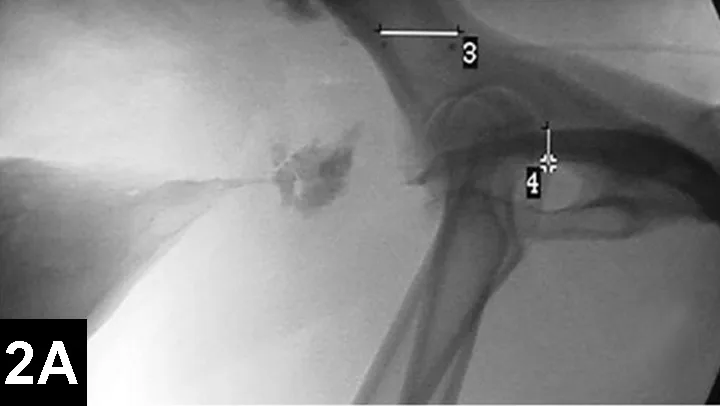
Figure 2A: Serial lateral positive contrast urethrocystograms in a dog with a malignant urethral obstruction secondary to a prostatic tumor.
Maximal urethral diameters measured using a colonic marker catheter to adjust for radiographic magnification.
Congenital Intrahepatic Portosystemic Shunts
While many of the techniques described here address clinical signs associated with obstructions of normal organs or lumens, IR techniques can also be used to close structures that are inappropriately open, such as vascular shunts, bleeding capillary beds (eg, hemorrhage of the nostrils), or tumor vascularization.
Numerous techniques have been described for attenuation of intrahepatic portosystemic shunts, but excessively high morbidity and mortality rates and cost have dissuaded many clients from pursuing open surgical therapy, despite overall superiority compared to medical management.
In IR, the goal for intrahepatic portosystemic shunts is to reduce the perioperative mortality rates associated with traditional open surgery and to improve outcomes (Figure 3). The author has recently reported 100 cases of intrahepatic portosystemic shunts in dogs that have been managed using IR techniques. Perioperative mortality rates were approximately 3%, and other complications were minor. The majority of these dogs have continued to do well following prolonged follow-up times.12
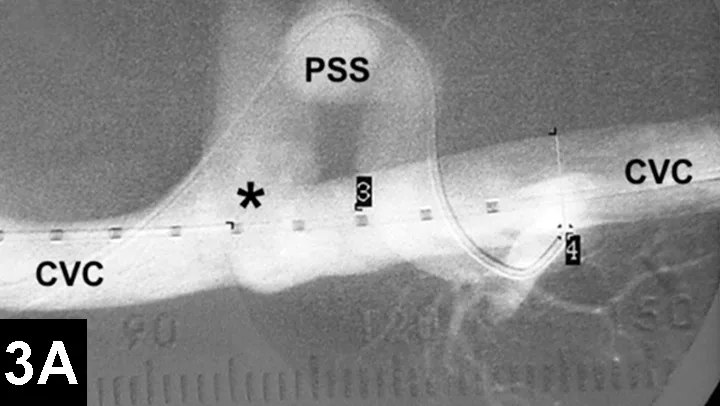
Figure 3A: Serial fluoroscopic images in a dog with a left divisional intrahepatic shunt.
The dog is in dorsal recumbency with the head to the left.
Double venogram of caudal vena cava (CVC) and portosystemic shunt (PSS) demonstrating entrance of shunt (*) into CVC.
Percutaneous Transarterial Embolization & Chemoembolization
Bland arterial embolization includes selective delivery of particulate material via catheter to occlude vascular malformations, control hemorrhage, or reduce tumor growth.
Chemoembolization involves selective delivery of intraarterial chemotherapy with subsequent particle embolization (Figure 4). Instead of administering systemic chemotherapy through a peripheral vein and exposing the patient to high systemic levels of these toxic drugs, local delivery into the arterial supply of the tumor has been shown to increase local drug concentrations, reduce systemic drug levels, reduce associated toxicities, and improve tumor response rates in humans with similar tumors.
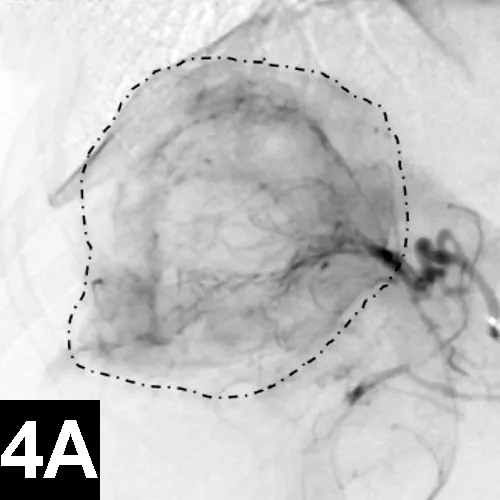
Figure 4A: Lateral digital subtraction angiograms of a dog with a nonresectable massive right-sided hepatocellular carcinoma.
The dog’s head is to the left.
Prechemoembolization angiogram demonstrating a massive liver tumor (surrounded by hyphenated line).
The author has performed this procedure in cases of invasive sinus carcinomas and unresectable hepatocellular carcinomas in dogs with encouraging results.13
When chemoembolization is performed in dogs, the author has documented as low as 1/20th the peak concentration of circulating chemotherapeutic agent found systemically as well as systemic chemotherapy exposure, which in turn theoretically reduces the toxic systemic side effects and subsequently results in improved tumor response rates when compared with intravenous administration.
Endourology
Similar techniques are currently being employed to manage ureteral obstructions secondary to uroliths, strictures, or malignancies (Figure 5). These procedures can be performed surgically or with minimal invasiveness (percutaneously or via cystoscopy) to reduce morbidity and improve outcomes in cats and dogs.9 For example, dogs with ureteral obstructions can now be treated through a small needle stick through the flank or endoscopically to relieve the obstruction and be discharged on an outpatient basis.
Currently, minimally invasive image-guided procedures can be performed on an outpatient basis to relieve these obstructions, provide urine drainage, improve patient comfort and renal function, and permit ongoing or subsequent chemotherapy administration to limit tumor growth and spread instead of simply euthanizing patients with malignant urinary tract obstructions.

Figure 5A: Serial lateral fluoroscopic images of a dog with ureteral obstruction secondary to trigonal transitional cell carcinoma.
Ultrasound-guided 18-gauge needle access into the renal pelvis with subsequent contrast ureteropyelogram demonstrating hydronephrosis and hydroureter (white arrows). A colonic marker catheter (black arrows) aids in radiographic magnification and stent length determination.
IO = interventional oncology, IR = interventional radiology