Canine Osteosarcoma: Part 1
Part 1 of this 2-part series on canine osteosarcoma provides a general overview, history, and diagnosis of the disease. Part 2 will describe treatment and follow-up protocols.
Profile
Definition
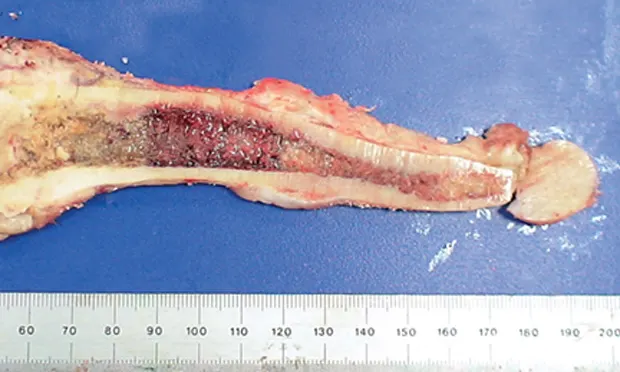
Osteosarcoma (OSA), a primary bone tumor comprised of neoplastic osteoblasts, can cause pain and lameness in affected limb(s) (Figure 1).* If left untreated, destruction of normal bone tissue can result in pathologic fracture.
OSA is an aggressive tumor with high risk for metastasis to the lungs and bone.
However, surgery and chemotherapy can typically extend the patient’s life longer than conservative (symptomatic) management.
Figure 1. Femur with osteosarcoma on sagittal section. The distal femur (left) has been largely replaced by the tumor.
Related Article: Commonly Used Chemotherapy Drugs - Part 1
Systems
OSA primarily affects the skeletal system but can occur in soft tissues or organs in some cases.
The primary OSA lesion most commonly occurs in the appendicular skeleton; thoracic limbs are more commonly affected than pelvic limbs.
Lesions may also occur in the ribs, skull, and other axial skeletal sites
Other bones distant from the primary lesion can also be affected by metastatic disease.
Metastatic disease is most commonly detected in the lungs.
At necropsy, metastasis to additional organs (eg, heart, liver, brain, spleen) may be evident.
Incidence & Prevalence
OSA, the most common primary bone malignancy in dogs, is responsible for the majority of skeletal system cancers.
Signalment
Breed Predilection
OSA is classically a cancer of large- and giant-breed dogs.
Breeds most at risk are the Saint Bernard, Great Dane, Irish setter, Doberman pinscher, rottweiler, German shepherd dog, and golden retriever.
Increased weight and height are the most predictive factors in dogs.
Small-breed dogs (<15 kg) account for ~5% of reported OSA cases; the majority of OSA in small-breed dogs is axial.
Age & Range
OSA is largely a disease of middle-aged to older dogs, with average age of onset of 7 years.
Small early peak in incidence may occur at 18–24 months of age.
OSA has been reported in dogs as young as 6 months of age.
Related Article: Advancing Oncology with Radiotherapy
Sex
Males are affected slightly more frequently than females (1.1–1.5:1).
In the Saint Bernard, rottweiler, Great Dane, and breeds with primary OSA of the axial skeleton (except rib and spine), affected females outnumber affected males.
Intact males and females reportedly have increased risk for OSA1; however, rottweiler males and females that underwent gonadectomy before 1 year of age had an approximate 1:4 lifetime risk for bone sarcoma and were significantly more likely than intact dogs to develop bone sarcoma.2
In this study, there was a markedly significant inverse dose-response relationship between duration of lifetime gonadal exposure and incidence of bone sarcoma independent of adult height or body weight.
To date, this has only been demonstrated in rottweilers.
Causes
The cause of canine OSA is currently unknown.
Related Article: Obtaining a Bone Biopsy
Risk Factors
Except for large body size, risk factors for OSA are unclear.
Because OSA tends to occur in major weight-bearing bones adjacent to late-closing physes, multiple minor traumas and subsequent injury to sensitive cells in the physeal region may occur.1
This may initiate the disease by inducing growth signals and increasing the probability of development of cancerous cells.
OSA has been associated with metallic implants used for fracture repair, chronic osteomyelitis, and fractures in which no internal repair was used.
Exposure to ionizing radiation can also induce OSA.
In humans exposed to nuclear waste, there is significantly higher prevalence of adult-onset OSA than in the general population.
An almost identical increased incidence of plutonium-induced OSA was reported in dogs exposed as young adults to plutonium or radium in experimental studies.
OSA is a rare, late-reported complication of radiation therapy in humans and dogs.
Signs
Signs of appendicular skeletal OSA are site dependent and include varying degrees of lameness, localized swelling, or mass effect in the affected limb.
In OSA of the skull, signs may include dysphagia (oral); exophthalmos and pain on opening the mouth (caudal mandibular or orbital); facial deformity and nasal discharge (sinus and nasal cavity); and hyperesthesia with or without neurologic signs (spinal).
Dogs with OSA of the ribs typically present with a palpable, variably painful mass; respiratory signs are uncommon even for lesions with large intrathoracic components.
Malignant pleural effusion is rare.
Dogs rarely have respiratory signs as the first evidence of pulmonary metastasis.
First signs are usually vague.
With radiographically detectable pulmonary metastasis, although dogs may appear subclinical for months, some develop nonspecific signs (eg, malaise, hyporexia) within 1–2 months.
Related Article: Bone Marrow Core Biopsy
Diagnosis
Definitive
Cytologic examination of a tumor specimen obtained by fine-needle aspiration is not thought to be definitive for OSA diagnosis.
However, this may support a tentative diagnosis, combined with radiographic appearance and clinical features, and provide confidence to discuss treatment options.
Alkaline phosphatase staining of cytologic samples may differentiate OSA from other sarcomas.
Lack of significant suppurative or pyogranulomatous inflammation on cytology may help rule out bacterial or fungal osteomyelitis.
In most cases, definitive diagnosis lies in procurement and interpretation of tissue for histopathology (ie, tissue biopsy).
Pros and cons of obtaining a pretreatment biopsy specimen (see Bone Biopsy) should be discussed with the owner so an informed decision about treatment can be made.
Benefits
Biopsy is the only method to definitively diagnose OSA before initiating such therapy as amputation.
Knowledge of specific tumor type may help avoid overextensive or inappropriate treatment of conditions that mimic OSA.
Disadvantages
Bone biopsy is not 100% diagnostic.
In approximately 10% of cases, the clinician may not obtain representative tissue, which can result in an inaccurate diagnosis.3
Bone biopsy may increase risk for pathologic fracture.
| Planning of the biopsy should include goals for hemostasis, wound closure, and asepsis. The incision must be small and in a location that can be completely excised with the tumor without compromising the limb-sparing procedure. Large or transverse incisions must be avoided.
A bone specimen may be obtained by closed needle-core (Jamshidi or bone marrow biopsy needle) or open trephine technique.
The advantage of an open technique is that a larger tissue sample can be obtained, which presumably improves the likelihood of establishing an accurate histologic diagnosis.
Postsurgical complications include hematoma formation, wound breakdown, infection, local seeding of tumor, and pathologic fracture.
Trephine biopsy is shown to have a diagnostic accuracy of 93.8%, but there is increased risk for pathologic fracture as compared with use of a smaller-gauge needle.4
The surgeon performing the definitive surgical procedure (especially for limb-sparing procedures) should also perform the preoperative bone biopsy.
Accuracy of diagnosis from smaller needle-core samples can depend on the technique, experience, and comfort level of the surgeon and pathologist.
Histopathology reports indicating the presence of reactive bone should not rule out a primary bone tumor or other pathology, especially if radiographic changes suggest a tumor.
In some cases, diagnosis can be difficult to establish by preoperative biopsy (ie, repeated biopsy attempts yield reactive bone); however, the pathologist should have no difficulty identifying a tumor when the entire specimen is available for histopathologic analysis (ie, postamputation).
This is likely because of heterogeneity of the tumor tissue and the large amount of reactive bone within the tumor, as well as whether the periphery vs the center of the tumor (center being more diagnostically accurate than periphery) was biopsied.
Obtaining a biopsy specimen using a Jamshidi needle has been shown to be accurate for detecting a tumor vs other disorders in 91.9% of cases and is accurate for diagnosis of specific subtypes of tumors in 82.3% of cases.5
|
Differential
Differentials of suspicious bone lesions identified on radiography include other primary bone tumors (ie, chondrosarcoma, fibrosarcoma, hemangiosarcoma), metastatic bone cancer, multiple myeloma or lymphoma of bone, systemic mycoses with bony localization, bacterial osteomyelitis, and rarely bone cysts.
Metastatic cancer can spread to bone from many other primary malignant sites.
Laboratory Findings
Minimum database should include CBC, serum chemistry panel, and urinalysis.
No laboratory findings are specific to OSA; however, the patient’s overall health status requires careful assessment.
Advanced age is not a contraindication for treatment, but prolonged anesthesia and chemotherapy may not be well tolerated in dogs with preexisting organ compromise.
Imaging
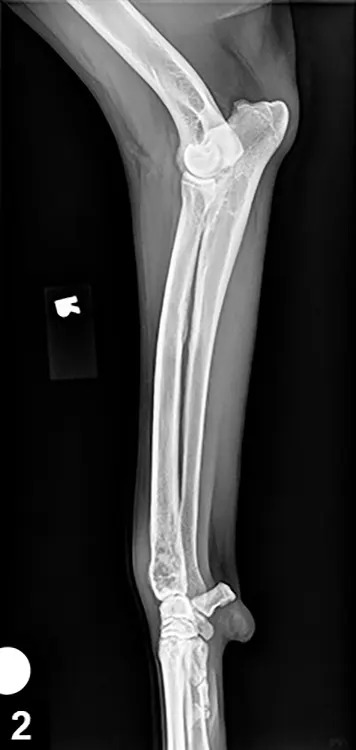
Evaluation of the primary site begins with good-quality radiography.
Overall radiographic features can vary from bone lysis to almost entirely osteoblastic changes (Figure 2. Radiographic features of OSA).
Cortical lysis is characteristic of OSA; severity may result in obvious areas of cortical discontinuity, resulting in pathologic fracture.
Soft tissue extension with obvious soft tissue swelling is often seen, and new bone (tumor, reactive bone) may form in a palisading pattern perpendicular to or radiating from the cortical axis (ie, sunburst pattern).
As the tumor invades the cortex, elevation of the periosteum occurs and new bone is laid down by the cambium layer, which provides triangular-appearing deposition of dense new cortical bone at the periphery of the lesion.
This periosteal new bone (referred to as Codman’s triangle) is not pathognomonic for OSA.
OSA rarely crosses articular cartilage.
Extension into periarticular soft tissues puts adjacent bones at risk.
Other radiographic changes include
Loss of the fine trabecular pattern in the metaphysis
Vague transition zone at the periphery of the medullary extent of the lesion instead of sharp sclerotic margin
Regions of fine punctate lysis
Lesion size, location, and duration will impact changes.
These radiographic changes may also be typical of other primary and metastatic bone tumors.
High-detail inspiratory thoracic radiographs should be obtained in an unsedated or unanesthetized patient to check for pulmonary metastatic disease.
Although some controversy exists, it is still considered important by most oncologists to include a VD or DV view and right and left lateral views.
Pulmonary metastases typically are not ossified and cannot be detected radiographically until the nodules are 6–8 mm in diameter.
It is uncommon to detect pulmonary metastatic disease at diagnosis (<10% of dogs).
Advanced imaging (eg, CT scan) has become more widely available and is considered more sensitive than plain radiography for detecting pulmonary metastasis.
Nuclear bone scan can be useful for detecting and localizing bone metastasis in dogs presenting with vague lameness or such signs as back pain.
The scan is sensitive but not specific for tumors.
Any region of osteoblastic activity, including osteoarthritis and infection, will have increased uptake of radiopharmaceutic agent.
Follow-up with high-detail radiography of sites that are suspicious on scintigraphy can generally help rule out nonneoplastic disease, but definitive biopsy may be necessary.
Additional OSA bone lesions detected on nuclear scintigraphy may represent either synchronous primary tumors or metastatic lesions and initally are often clinically silent.
OSA = osteosarcoma
Nicole Ehrhart, VMD, MS, DACVS, is a professor of surgical oncology at Colorado State University. She completed an internship in general medicine and surgery at the Animal Medical Center in New York City and was accepted into a surgery residency at Colorado State University. She completed her surgical residency and master’s degree in clinical sciences and became board certified in surgery during the first year of a 2-year postdoctoral Fellowship in Surgical Oncology and Orthopedic Research. Dr. Ehrhart graduated from the University of Pennsylvania School of Veterinary Medicine.