Ammonium Urate Urolithiasis
Gregory F. Grauer, DVM, MS, DACVIM (SAIM), Kansas State University

How do I manage ammonium urate urolithiasis?
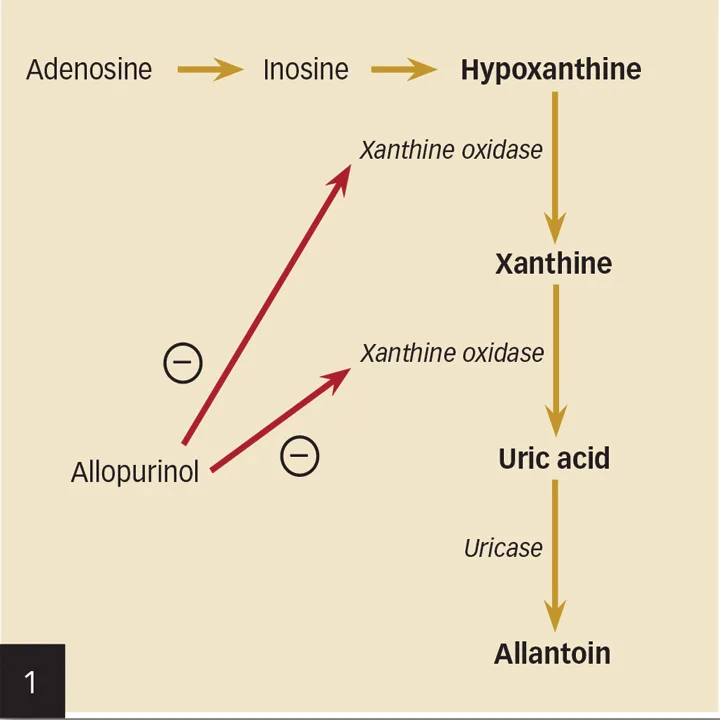
Formation of urate uroliths (eg, ammonium urate [AU], uric acid, calcium urate, sodium urate) occurs from urine supersaturation with purine metabolites. Uric acid derives from the metabolic degradation of endogenous purine ribonucleosides and dietary nucleic acids (Figure 1) and is typically converted by uricase within hepatocytes to allantoin. Compared with uric acid and xanthine, allantoin is relatively soluble in urine.
Metabolism of AU uroliths. Image courtesy Mal Rooks, CMI
Decreased proximal tubular reabsorption of uric acid increases uric acid and sodium urate concentrations in urine. Hyperuricosuria is a risk factor for—but not the only cause of—urate urolith formation; the definitive mechanism(s) remains to be determined.
Urate uroliths most commonly form in younger dogs (mean age, 3–4 years) and are usually small, smooth, round or ovoid, and green or brown (Figure 2). They are relatively radiolucent and may require ultrasonography or contrast radiography to be observed (Figures 3–6). Urinary tract infections (UTIs), especially those with urease-producing bacteria, may facilitate ammonium acid urate crystallization. UTI may also occur secondary to urolith-induced mucosal damage and altered local host defense mechanisms. High dietary protein usually increases the urinary excretion of both UA and ammonium ions in affected dogs. Ammonia, produced by renal tubular cells from glutamine, diffuses into the tubular lumen and serves as a buffer for secreted hydrogen ions, thereby forming ammonium ions. Ammonium ions are relatively lipid insoluble and become trapped within the tubular fluid. Acidic urine decreases the solubility of uric acid. AU crystals are usually yellow–brown and spherical with multiple irregular protrusions and are often referred to as thorn apple crystals (Figures 7 & 8).

Figure 2.
Typical appearance of canine AU uroliths (scale, 1 division = 1 mm).
Urate uroliths may form in dogs with hepatic insufficiency (eg, hepatic cirrhosis, microvascular dysplasia, portosystemic shunt [PSS]) as a result of increased renal excretion of AU.5 PSSs are common in the miniature schnauzer, Yorkshire terrier, West Highland white terrier, and Pekingese, making ammonium urate uroliths more common in these breeds.6,7 Male and female dogs are equally affected, and uroliths commonly form before 3 years of age.4 Urate uroliths in dogs without a known genetic predisposition should prompt an evaluation of liver function with serum bile acids.
Urate uroliths account for 3% to 10% of feline uroliths,8,9 but the pathogenesis is incompletely understood. Urate uroliths can form in cats with PSS. In addition, genetics may be involved as the Siamese, Egyptian Mau, and Birman breeds appear to be predisposed.8,9 Cats with PSS tend to form urate uroliths at a younger age than cats with normal hepatic function (2 vs 7 years).8 Urate uroliths in cats are relatively radiolucent, and urate crystalluria is uncommon.
Xanthine uroliths can occur spontaneously (most common mechanism in cats) or in association with the use of allopurinol (eg, for dissolution/prevention of urate uroliths, treatment of leishmaniasis [most common mechanism in dogs]). Xanthinuria and xanthine uroliths have been observed in the Cavalier King Charles spaniel independent of allopurinol treatment.10
Management in Dogs
Urate uroliths in dogs may be removed mechanically (eg, voiding hydropropulsion, catheter retrieval, surgery) or dissolved medically. Small, multiple, relatively radiolucent urate uroliths that often recur can be difficult to visualize, making radiographic monitoring and surgical management challenging. Medical dissolution of urate uroliths not associated with PSS should include a diet low in protein and nucleic acids, alkalinization of the urine, xanthine oxidase inhibition, and elimination of UTI.11 Hill’s Prescription Diet u/d Canine Non-Struvite Urinary Tract Health has reduced protein and purine content and produces alkaline urine; therefore, it is recommended for the dissolution and prevention of urate uroliths.11 The reduced protein content decreases the hepatic formation of urea and hence decreases renal medullary hypertonicity and urine-concentrating ability.
In addition, allopurinol, a competitive inhibitor of the enzyme xanthine oxidase (Figure 9, see gallery), which converts hypoxanthine to xanthine and xanthine to uric acid (Figure 1, see gallery), should be administered in dogs at a dose of 10 mg/kg PO q8h. If necessary, potassium citrate should also be administered to maintain a urine pH of 7.0 to 7.5; the dose should be individualized, with dosages starting at 40 to 75 mg/kg PO q12h. UTI should be appropriately treated because urease-producing organisms increase the urine ammonium ion concentration and potentiate AU crystal production.
Side effects of allopurinol in humans include vomiting, rash, leukopenia, thrombocytopenia, vasculitis, and hepatitis; these events have been reported only rarely in dogs.3 Allopurinol is excreted via the kidneys and thus should be used cautiously in azotemic dogs. Xanthine uroliths may form in dogs treated with allopurinol, especially if dietary protein and purine intake are not reduced.
Recurrence rates for urate uroliths in all dog breeds are high (33%–50%), and uroliths usually recur within 1 year of initial diagnosis and treatment.12 Prophylactic dietary therapy is the first line of defense. Diets recommended to prevent recurrence in dogs include Royal Canin Veterinary Diets Canine Urinary UC Low Purine, Purina Veterinary Diets HA Hypoallergenic Canine Formula, and Hill’s Prescription Diet u/d Canine Non-Struvite Urinary Tract Health. If urate crystalluria persists despite good dietary compliance, urine pH should be monitored to assure appropriate alkalinization and potassium citrate administered if needed. If necessary (ie, xanthine crystals detected in urine sediment), lower doses of allopurinol (10–15 mg/kg q24h) may be used.3 The risk of inducing xanthine uroliths increases with higher doses of allopurinol as well as with higher dietary protein and purine, and caution is warranted with long-term use of high-dose (15 mg/kg PO q12h) allopurinol.
In dogs with urate urolithiasis secondary to severe hepatic insufficiency, the underlying disorder should be corrected if possible. If hepatic function can be improved (eg, surgical or medical management of a PSS) and the urine becomes undersaturated with ammonium and urate ions, uroliths may dissolve spontaneously; nevertheless, urolith removal via cystotomy at PSS correction or associated with medical management is usually recommended, especially in male dogs, to reduce the likelihood of urethral obstruction. In dogs with inoperable PSS or microvascular dysplasia, medical management including diet, antibiotics, and lactulose may be used to help decrease urine saturation with AU and reduce signs of hepatic encephalopathy.
Management in Cats
Management of urate uroliths in cats is similar to that in dogs, although dissolution protocols have not yet been developed. In cats with PSS, correction and/or medical management of the shunt should be the first priority. Subsequent to surgical removal of urate uroliths, diets lower in protein and purine that maintain a urine pH of at least 6.6 are recommended. Canned foods are recommended to decrease urine concentration of calculogenic substances. Potassium citrate (25–75 mg/kg PO q12–24h) should be administered if urine pH is consistently below 6.6. Although allopurinol has been used in conjunction with diet at the University of Minnesota to medically dissolve feline urate uroliths, its use in cats to dissolve or prevent urate uroliths cannot be routinely recommended until the results of further studies are available.3 Diets recommended to prevent recurrence of feline urate uroliths include Purina Veterinary Diets NF Kidney Function Feline Formula, Iams Veterinary Formula Renal Multi-Stage Renal/Feline, Royal Canin Veterinary Diet Feline Modified Renal LP, Hill’s Prescription Diet k/d Feline Renal Health, and Hill’s Prescription Diet l/d Feline Hepatic Health.
In Closing
Monitoring dogs at risk for AU urolith recurrence should include close monitoring for signs of lower urinary tract inflammation or urethral obstruction, as well as periodic evaluation of urine pH and urine sediment for crystalluria. Any UTIs should be treated on the basis of urine culture and sensitivity testing. Recurrence of AU uroliths can be confirmed with imaging which may require ultrasonography or contrast radiography.
The Dalmation Dilemma
In the dalmatian, a defect of the uric acid transporter SLC2A9 alters hepatic and renal transport of uric acid,1 leading to abnormally high serum and urine concentrations of uric acid2; the dalmatian typically has a serum uric acid concentration 2–4 times higher than in other breeds.3 The English bulldog, Parson Russell terrier, and black Russian terrier may also have the transporter defect. The mutation is a simple autosomal recessive trait for which affected individuals are homozygous. A DNA test is available to confirm the defect: vgl.ucdavis.edu/services/Hyperuricosuria.php.
Although urinary uric acid excretion is approximately 10 times higher in the dalmatian than in other dogs, urate uroliths form in only a small percentage (approximately 5%) of dalmatians.4 Approximately 60% of urate uroliths occur in dalmatians, and, conversely, approximately 90% of the uroliths in dalmatians are urates.3 The male:female ratio for urate urolith formation in the dalmatian is approximately 13:14; for all breeds, more than 9 of 10 urate uroliths submitted to the Minnesota Urolith Center are from male dogs.3
AU = ammonium urate, PSS = portosystemic shunt, UTI = urinary tract infection